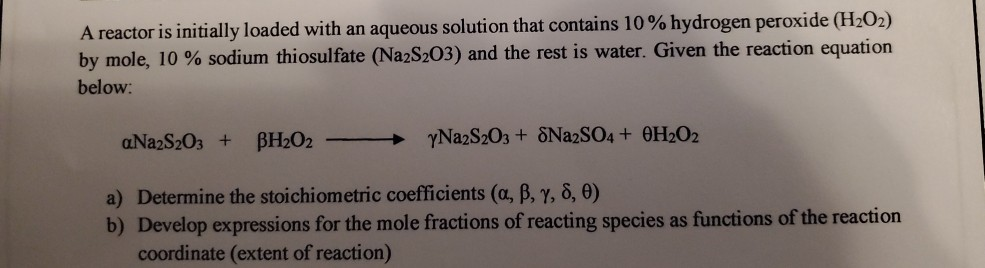
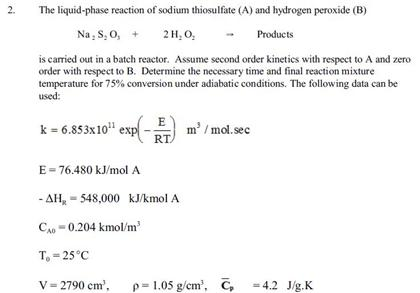
SOLVED: Background: This oscillating clock examines the reaction between iodide, hydrogen ion, and hydrogen peroxide. The reaction order of hydrogen peroxide will be shown to be first order, and the rate constant (
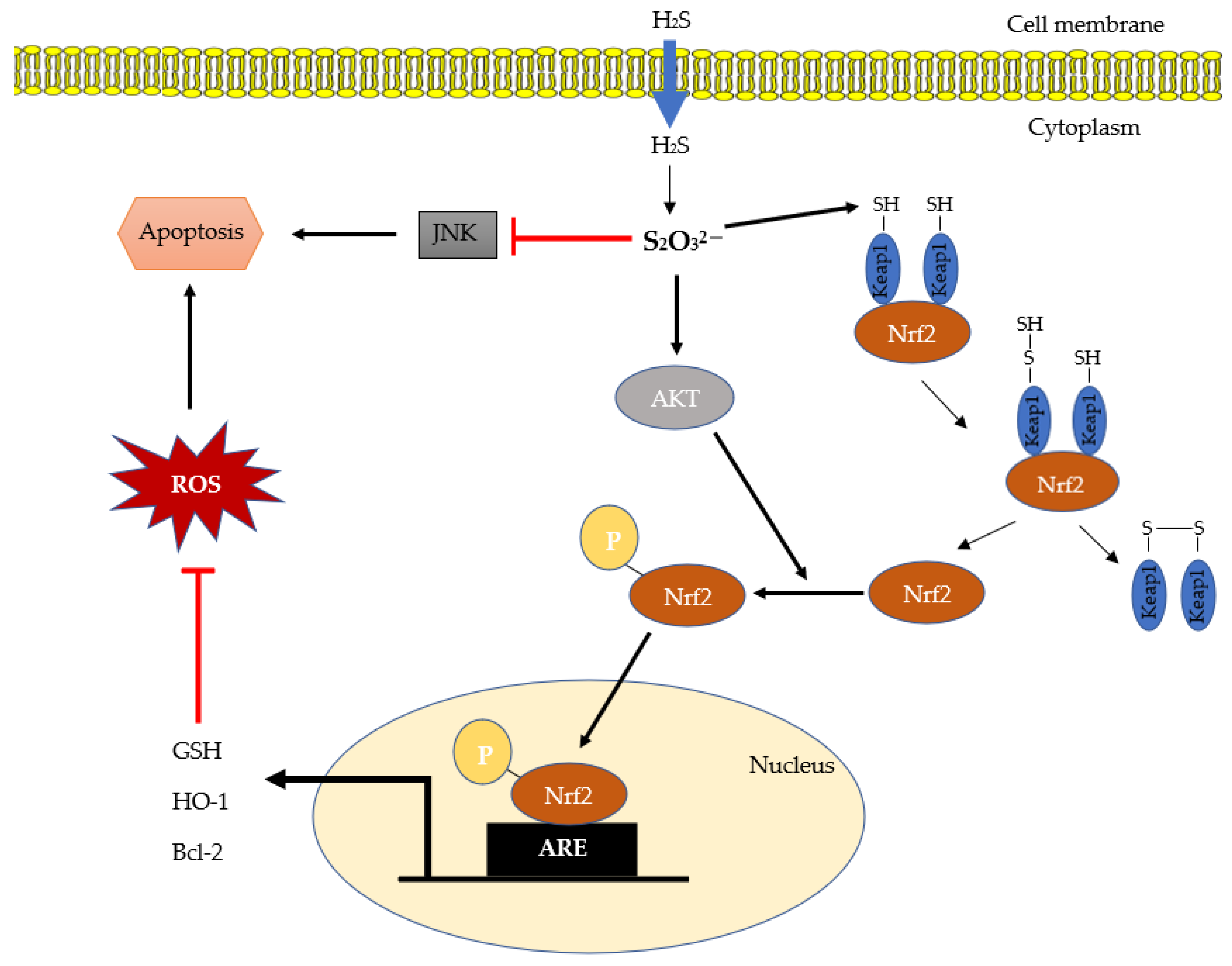
IJMS | Free Full-Text | Hydrogen Sulfide Metabolite, Sodium Thiosulfate: Clinical Applications and Underlying Molecular Mechanisms

SOLVED: What is the role of the thiosulfate in this experiment " Oxidation of Iodide with hydrogen peroxide " ?

Amazon.com: LabChem LC250604 Sodium Thiosulfate Solution, 0.1N (0.1M), 4 L Volume : Industrial & Scientific
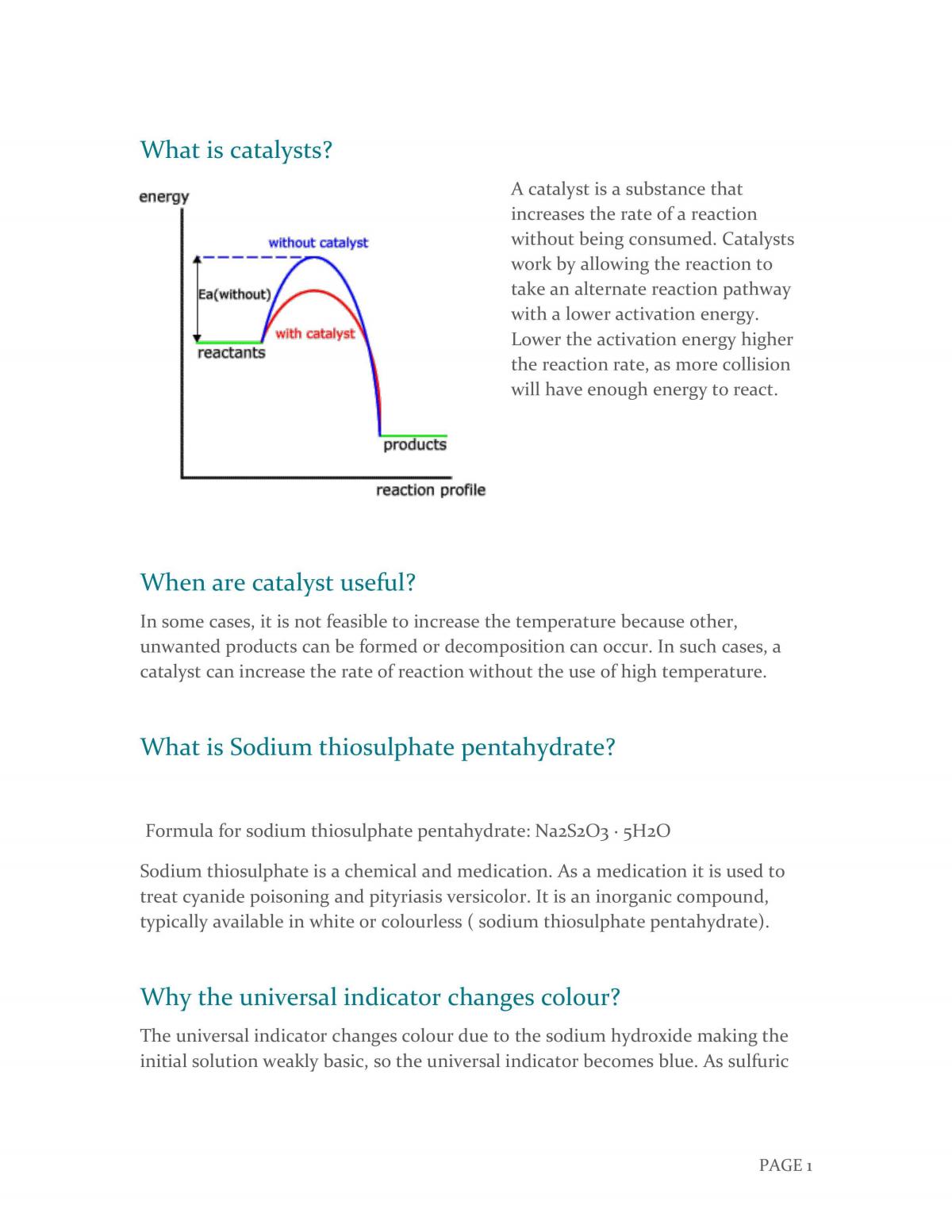
Catalysis of the Reaction Between Sodium Thiosulphate Pentahydrate and Hydrogen Peroxide | Chemistry - Year 11 HSC | Thinkswap
The use of sodium thiosulfate for inactivating residual hydrogen peroxide on contact lenses after disinfection: Clinical and Experimental Optometry: Vol 69, No 1

Chlorine is preferred over bisulfite for H2O2 quenching following UV-AOP drinking water treatment - ScienceDirect

Ch. 20 Applications of oxidation/reduction titrations 1.Auxiliary oxidizing and reducing reagents Fe(II), Fe(III) - Auxiliary reducing reagents A number. - ppt download

Mechanism of the oxidation of thiosulfate with hydrogen peroxide catalyzed by aqua-ethylenediaminetetraacetatoruthenium(III) - ScienceDirect

A 2.00 mL sample of an aqueous solution of hydrogen peroxide, H2O2(aq), is treated with an excess of Kl(aq). The liberated I2 requires 12.40 mL of 0.1025 M Na2S2O3 for its titration.




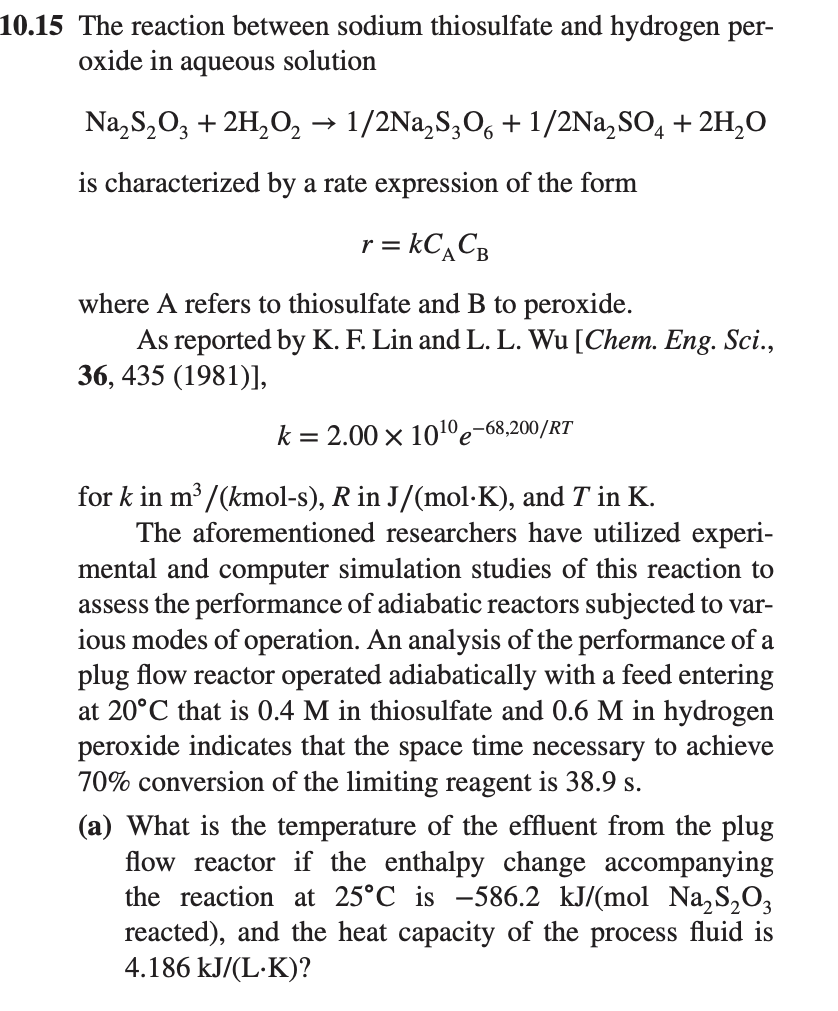
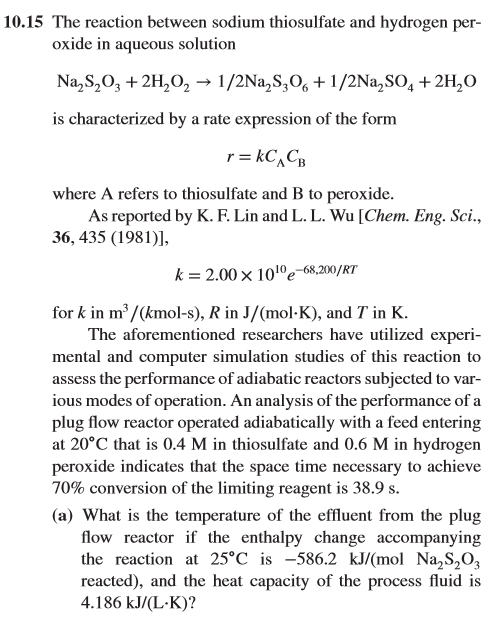
![Solved 4. [Problem 10-15, pp 332-333 in Hill.] The reaction | Chegg.com Solved 4. [Problem 10-15, pp 332-333 in Hill.] The reaction | Chegg.com](https://media.cheggcdn.com/media%2F9cb%2F9cbf32aa-82aa-4a8d-b31c-3211357af8eb%2Fphpkupd3t.png)