Pellets and gummies: Seeking a 3D printed gastro-resistant omeprazole dosage for paediatric administration - ScienceDirect
Implementation of the 11th Edition of European Pharmacopoeia – Notification for CEP holders - European Directorate for the Quality of Medicines & HealthCare
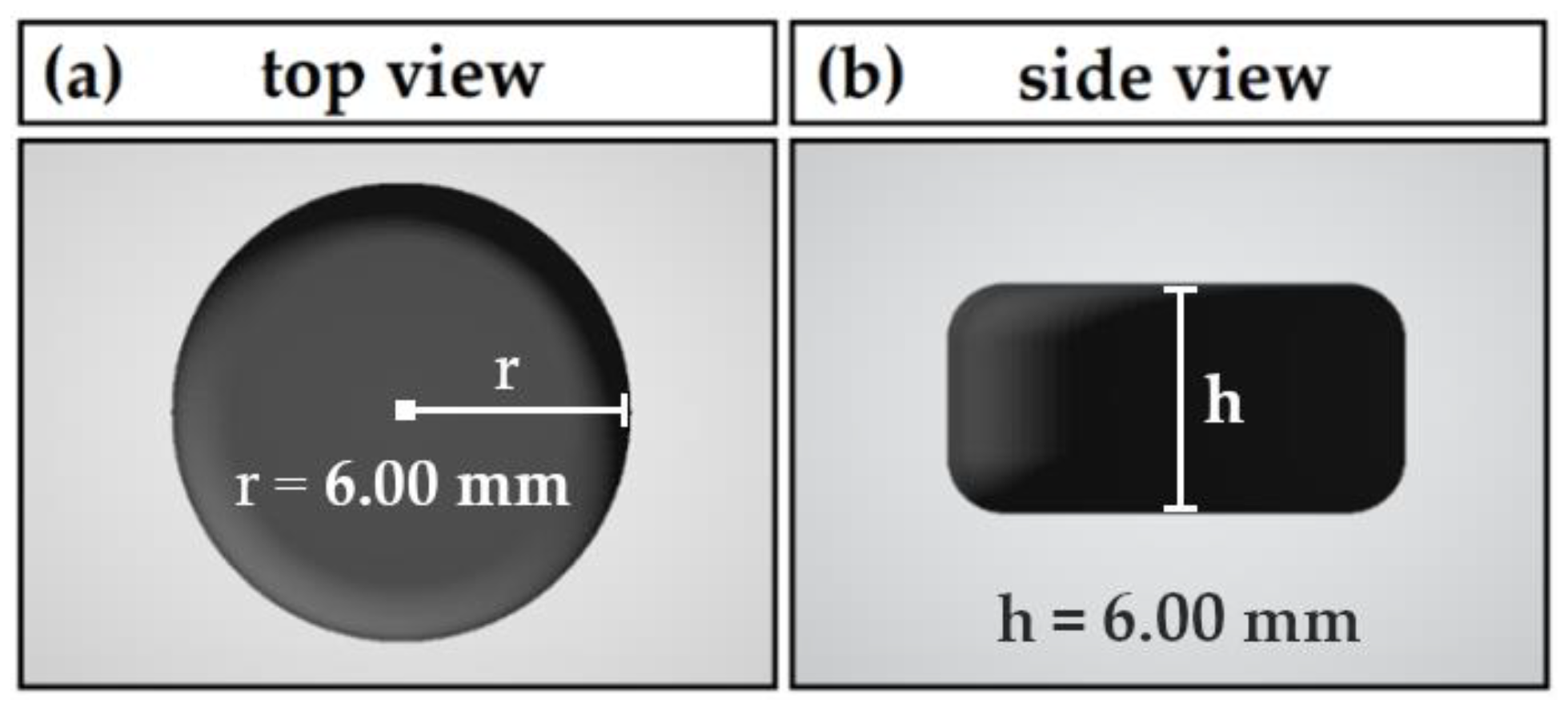
Pharmaceutics | Free Full-Text | Novel Approach to Pharmaceutical 3D-Printing Omitting the Need for Filament—Investigation of Materials, Process, and Product Characteristics
PDF) How Granular Can a Dose Form Be Described? Considering EDQM Standard Terms for a Global Terminology

European Pharmacopoeia (Ph. Eur.) 11th Edition - European Directorate for the Quality of Medicines & HealthCare

European Pharmacopoeia (Ph. Eur.) 11th Edition - European Directorate for the Quality of Medicines & HealthCare
Ph. Eur. Reference Standards: Orders and Catalogue - European Directorate for the Quality of Medicines & HealthCare
Ph. Eur. publishes key harmonised monographs on Paraffin, white soft and Paraffin, yellow soft - European Directorate for the Quality of Medicines & HealthCare
7 new reference standards and 26 replacement batches released in April 2021 - European Directorate for the Quality of Medicines & HealthCare

Ph. Eur. Reference Standards: Orders and Catalogue - European Directorate for the Quality of Medicines & HealthCare