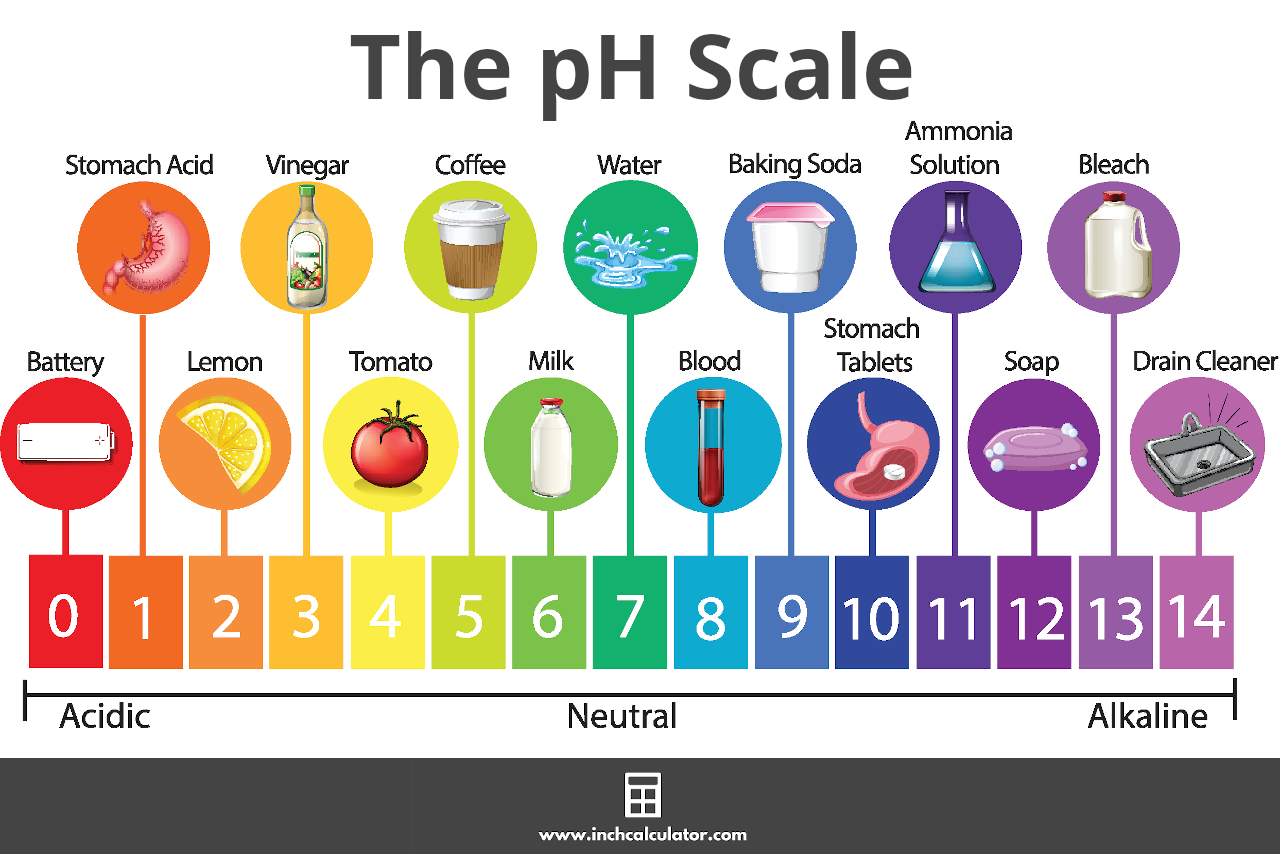
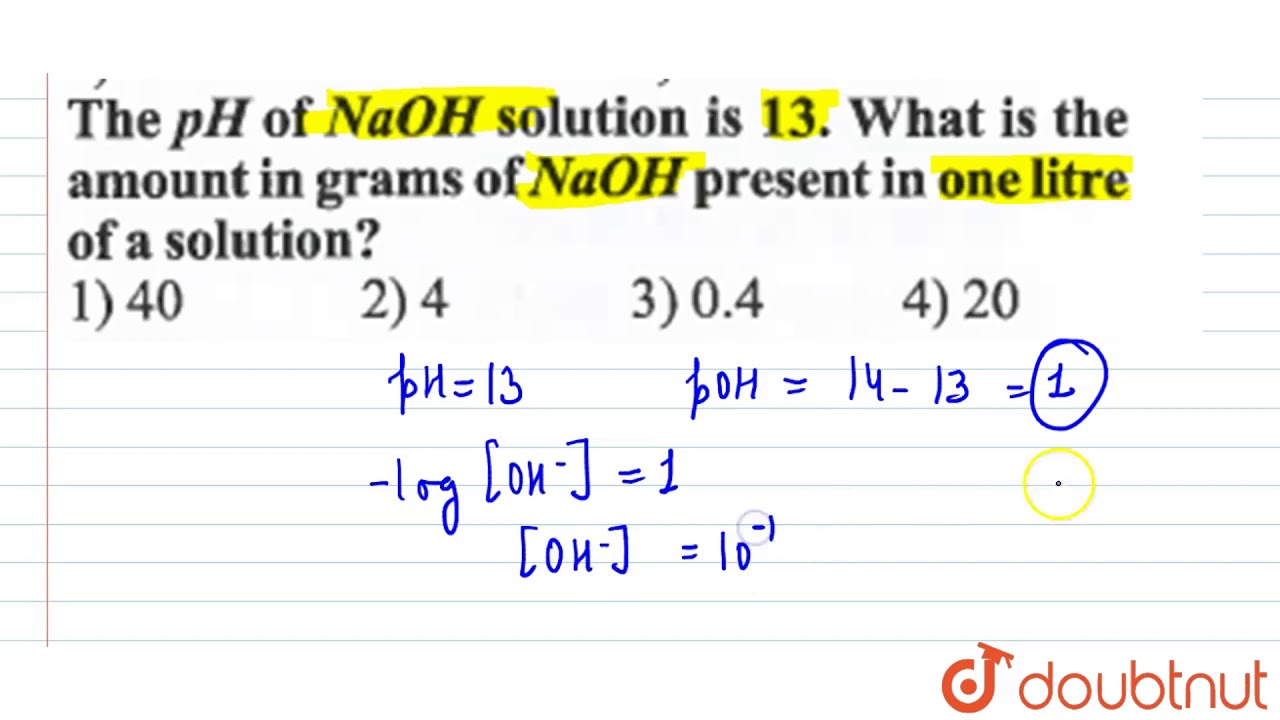
If an acetic acid solution is titrated with NaOH, how do I find out the amount of NaOH needed so the pH of the solution is exactly 7? Because the pH at

SOLVED: 25 mLof0.15 M benzoic acid (K 6.4*10*5) is titrated by Calculate the pH of the 0.85 M NaOH: acid solution before = any titrant is added. pH Calculate the pH after
![PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download PH calculations. What is pH? pH = - log 10 [H + (aq) ] where [H + ] is the concentration of hydrogen ions in mol dm -3 to convert pH into hydrogen ion. - ppt download](https://images.slideplayer.com/24/7441095/slides/slide_4.jpg)
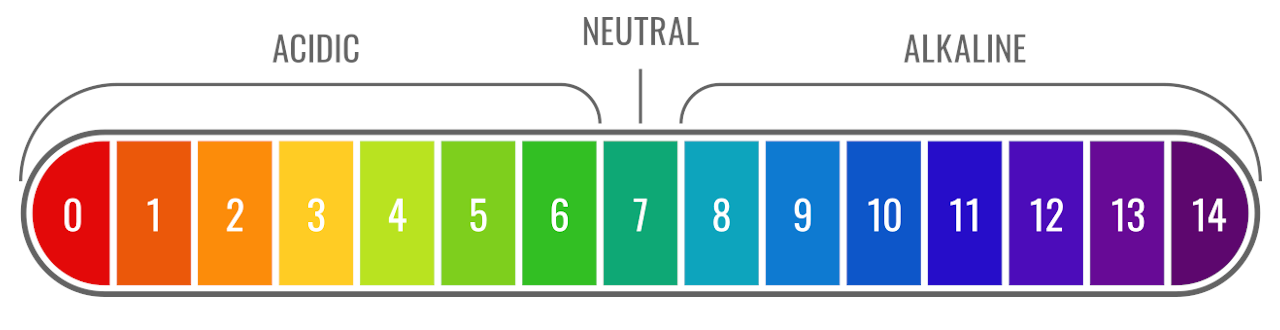
![Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE - YouTube Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE - YouTube](https://i.ytimg.com/vi/gn1CgBzShps/maxresdefault.jpg)



![Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and Bases - Practice - YouTube Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and Bases - Practice - YouTube](https://i.ytimg.com/vi/UiK37I159fc/maxresdefault.jpg)