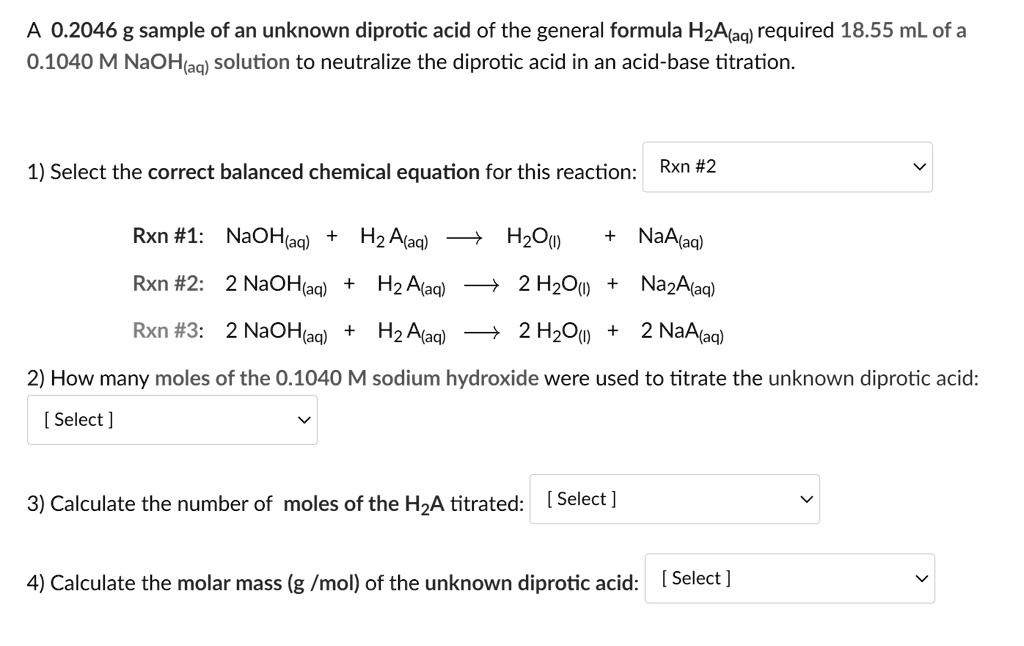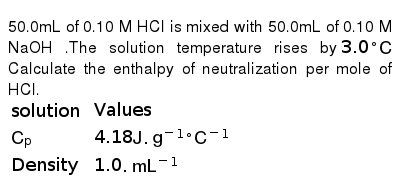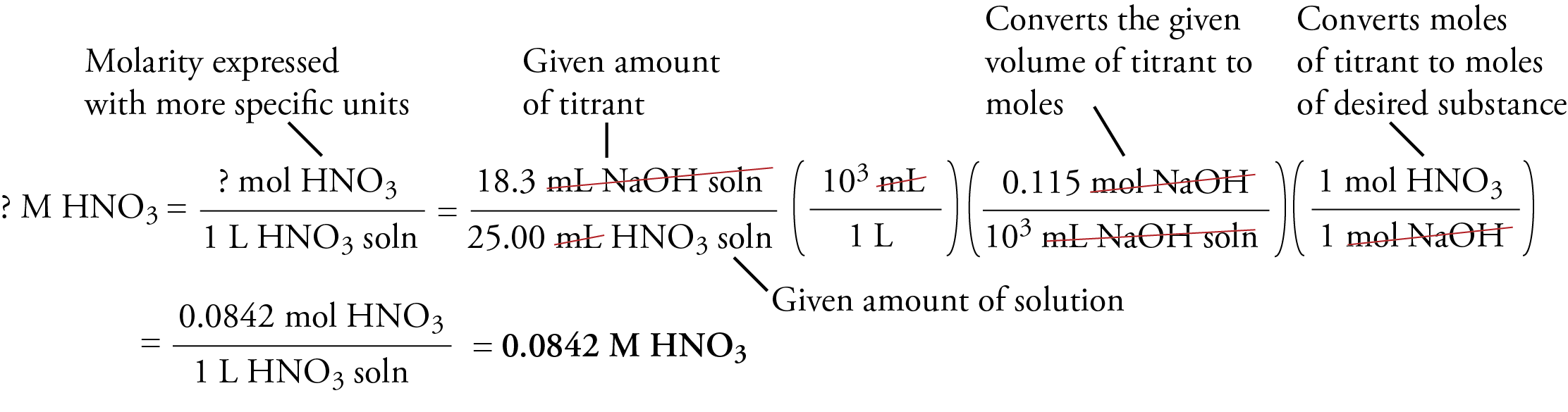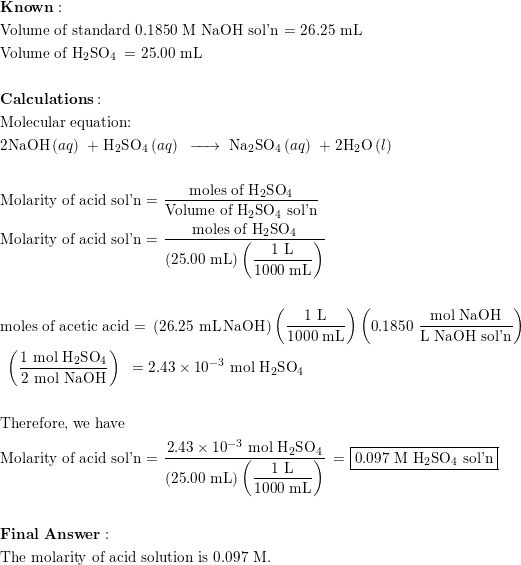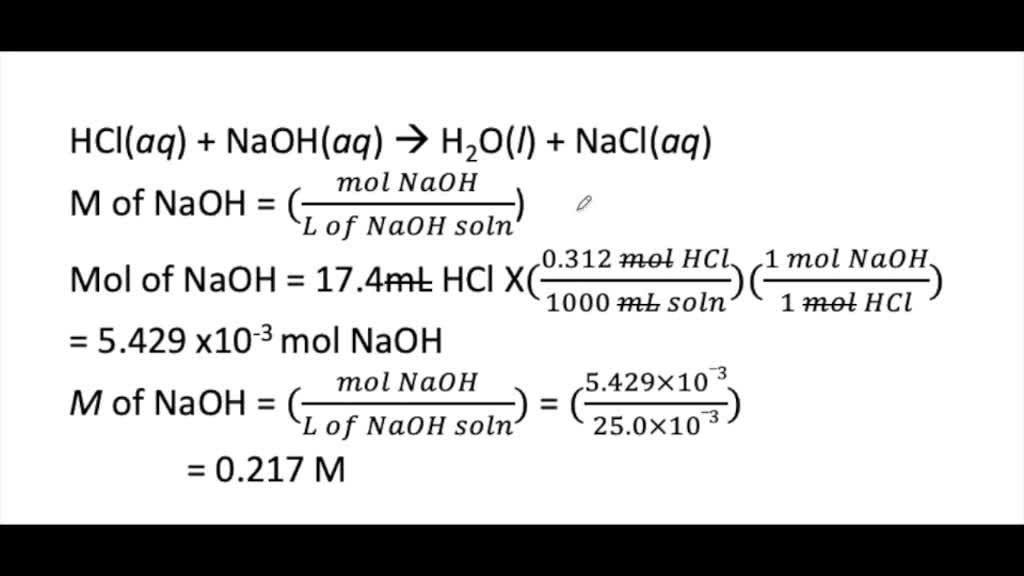
SOLVED:Calculate the concentration (in molarity) of an NaOH solution if 25.0 mL of the solution is needed to neutralize 17.4 mL of a 0.312 M HCl solution.

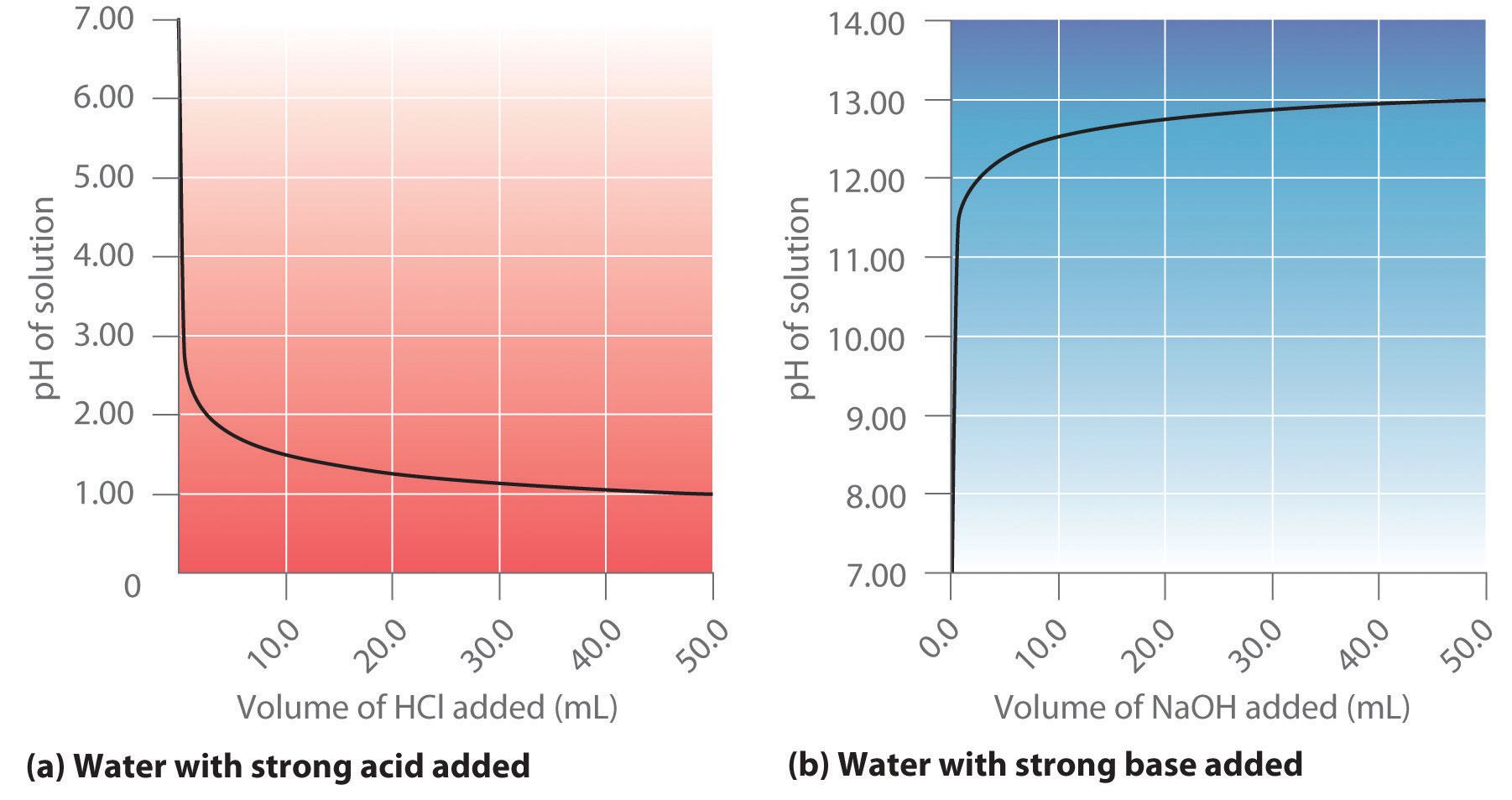
Acid-Base Reactions. Neutralization acid + base salt + water HCl (aq) + NaOH (aq) NaCl (aq) + H 2 O (l) H + + Cl - + Na + + OH - Na + + Cl - + H 2 O (l) - ppt download
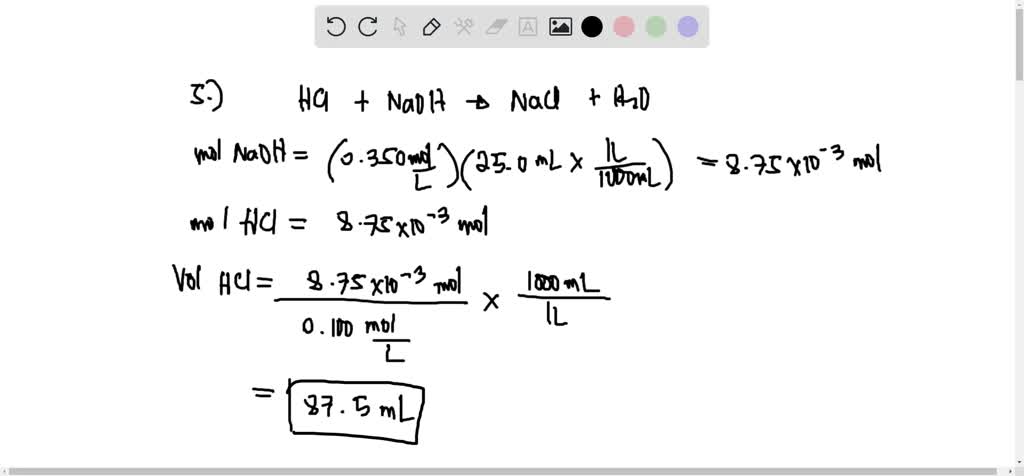
SOLVED: 5. Calculate the volume of 0.100 M HCI solution needed to neutralize 25.0 mL of 0.350 M NaOH solution. (Answer: 87.5 mL) Calculate the volume of 0.100 M HzSO4 solution needed
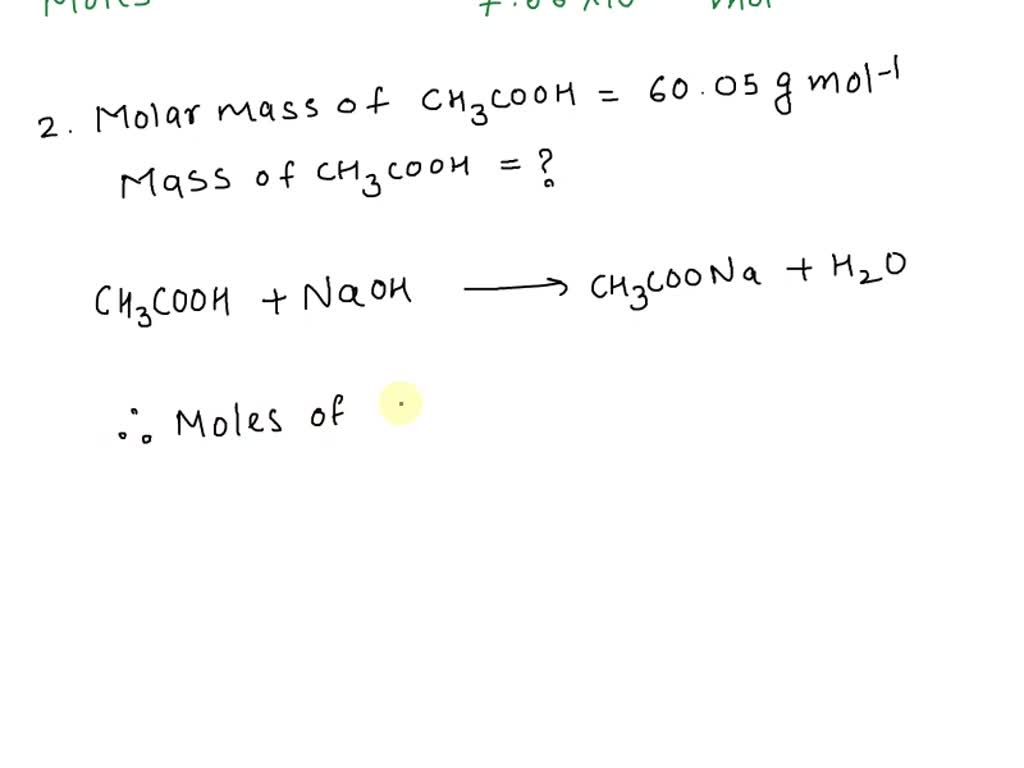
SOLVED: 1) In a titration of vinegar against sodium hydroxide solution, exactly 74.80 mL of 0.1024M NaOH was needed to neutralize the acetic acid contained in the vinegar. Calculate the number of
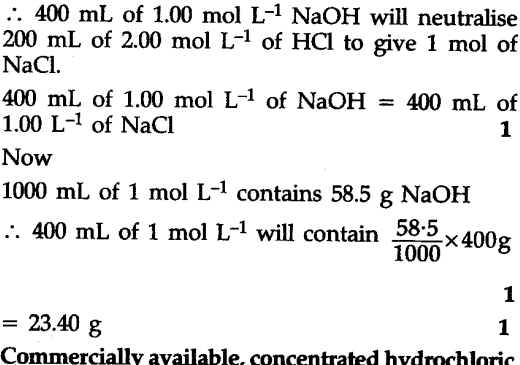
Calculate the volume of 1.00 mol ${{L}^{-1}}$ aqueous sodium hydroxide that is neutralised by 200 mL of 2.00 mol ${{L}^{-1}}$ aqueous hydrochloric acid arid the mass of sodium chloride produced - CBSE

the volume of 05 molars naoh solution required for complete neutralization of 365 gram hcl is gi4mkkoo -Chemistry - TopperLearning.com

Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.

Neutralizing Solutions with Sodium Hydroxide | Process & Chemical Formula - Video & Lesson Transcript | Study.com

Enthalpy of neutralisation of acetic acid by NaOh is -50.6 kJ mol^(-1). Calculate DeltaH for ionisation of CH(3)COOH. Given. The heat of neutralisation of a strong acid with a strong base is -