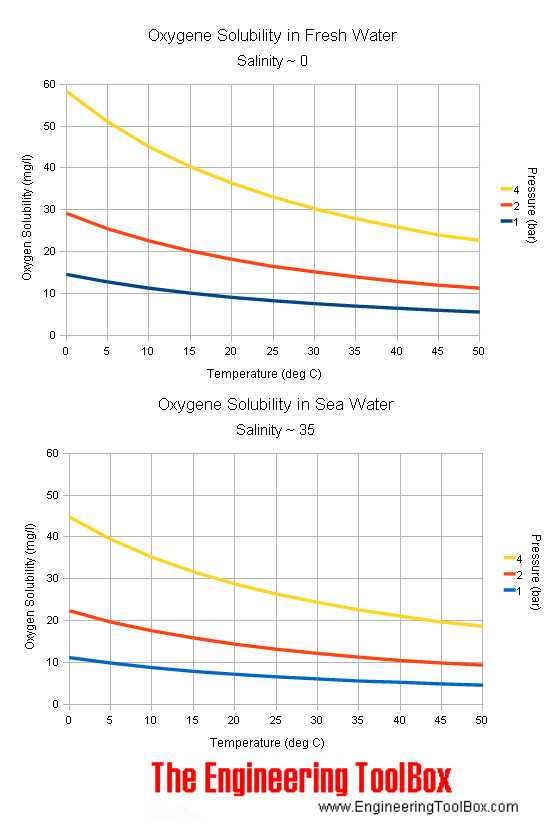
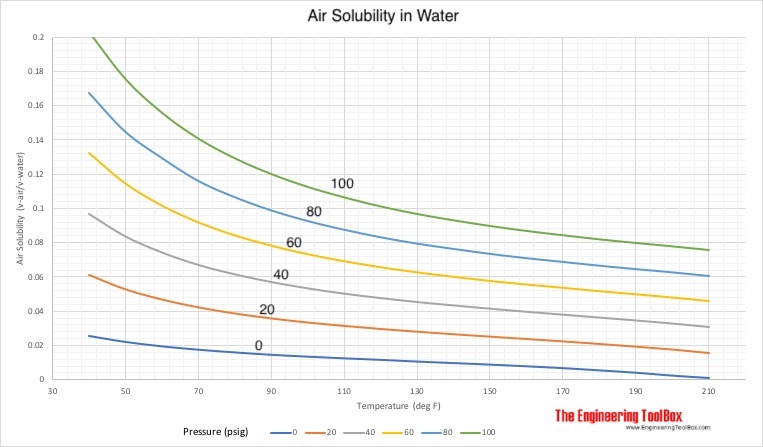
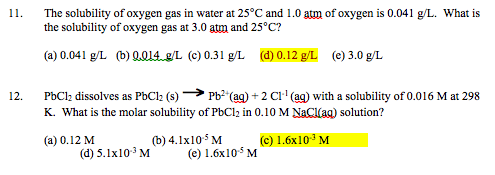
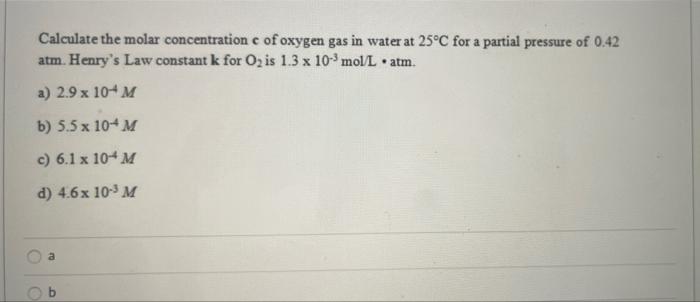
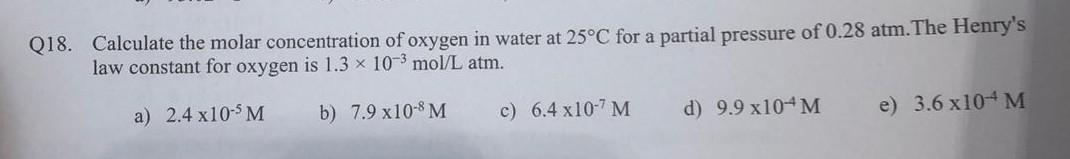SOLVED: Enter your answer in the provided box Calculate the molar concentration of oxygen in water at 259€ for partial pressure of 0.H4 atm . The Henry'haw constant for oxygen is 13

Henry's law constant for oxygen dissolved in water is 4.34 × 10^4 atm at 25^C . If the partial pressure of oxygen in air is 0.4atm. Calculate the concentration (in moles per
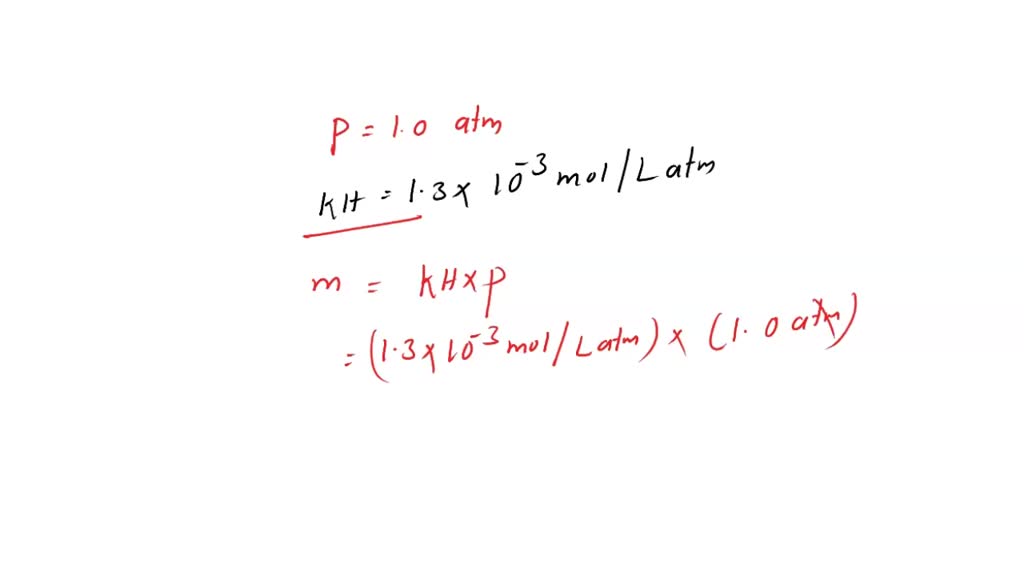
SOLVED: What is the molar concentration of oxygen in water at 25C for a partial pressure of 1.0 atm (KH = 1.3 x 10-3 mol/Latm)

SOLVED: Calculate the molar concentration of oxygen in water at 25°C for a partial pressure of 0.24 atm. The Henry's law constant for oxygen is 1.3 × 10−3 mol/L · atm. Report

Henry's law constant for oxygen dissolved in water is 4.34 × 10^4 atm at 25^C . If the partial pressure of oxygen in air is 0.4atm. Calculate the concentration (in moles per

What is the concentration of O_2(g) in water at 25 degree C exposed to a partial pressure of oxygen of 325 mmHg? The Henry's law constant for oxygen gas at 25 degree

Henry's law constant for oxygen dissolved in water is 4.34 × 10^4 atm at 25^C . If the partial pressure of oxygen in air is 0.4atm. Calculate the concentration (in moles per
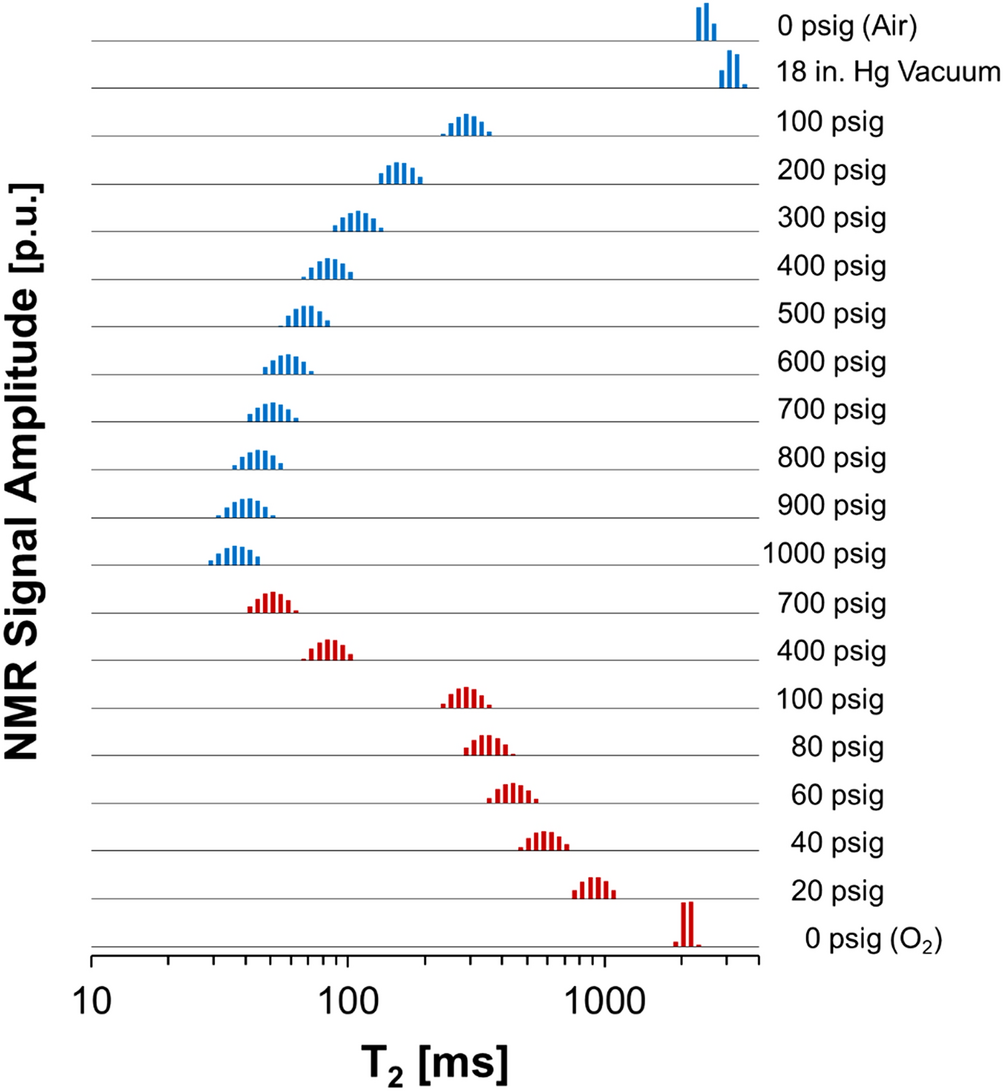
Quantification of dissolved O2 in bulk aqueous solutions and porous media using NMR relaxometry | Scientific Reports
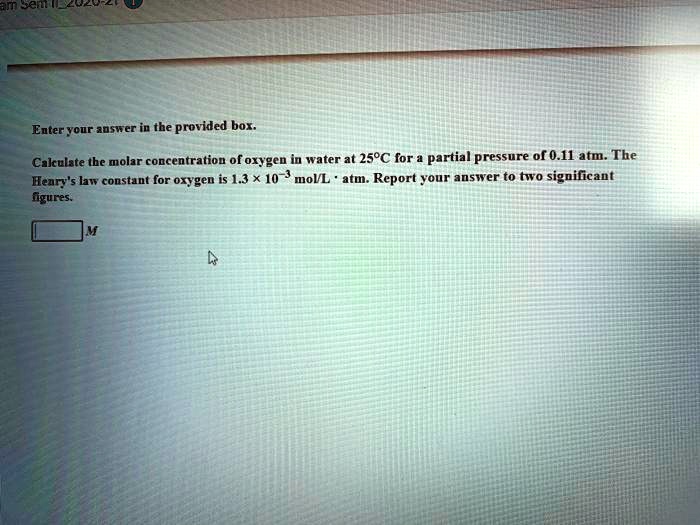
SOLVED: Enter your answer in the provided box. Calculate the molar concentration of oxygen in water at 25C for a partial pressure of 0.11 atm.The Heary's law constant for oxygen is 1.3